Understanding the concept of valence electrons is crucial in chemistry, as it helps in predicting the chemical properties and behavior of elements. The HCN molecule, composed of hydrogen, carbon, and nitrogen, is a fascinating example to explore when discussing valence electrons. Here, we will delve into the world of HCN valence electrons, providing 5 key tips to enhance your comprehension of this subject.
Key Points
- Valence electrons play a critical role in determining the chemical reactivity of HCN.
- The molecular structure of HCN influences the distribution of valence electrons among its atoms.
- Understanding the valence electrons in HCN can help predict its potential reactions and interactions with other molecules.
- The electronegativity of nitrogen and carbon affects the distribution of valence electrons in the HCN molecule.
- Valence electrons are crucial for understanding the molecular orbital theory and its application to HCN.
Introduction to HCN Valence Electrons

The HCN molecule, or hydrogen cyanide, is a compound that consists of one hydrogen atom, one carbon atom, and one nitrogen atom. To understand the valence electrons in HCN, we must first know the atomic number of each element involved: hydrogen (H) has an atomic number of 1, carbon © has an atomic number of 6, and nitrogen (N) has an atomic number of 7. The valence electrons are the electrons in the outermost shell of an atom, which participate in chemical bonding.
Determining Valence Electrons in HCN
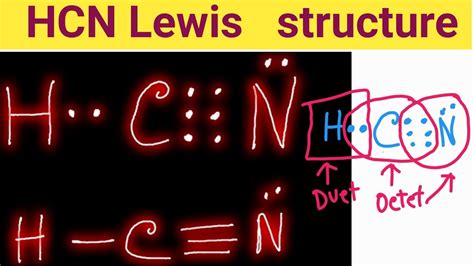
To determine the number of valence electrons in each atom of the HCN molecule, we look at their electron configurations. Hydrogen has 1 valence electron, carbon has 4 valence electrons, and nitrogen has 5 valence electrons. When these atoms form a molecule, they share or exchange electrons to achieve a stable electronic configuration, often aiming to have a full outer shell, similar to the noble gas configuration.
In the case of HCN, the molecule is formed through covalent bonds between the hydrogen, carbon, and nitrogen atoms. The carbon and nitrogen atoms form a triple bond, which includes one sigma (σ) bond and two pi (π) bonds, while the hydrogen atom forms a single sigma bond with the carbon atom. The sharing of electrons in these bonds involves the valence electrons of the atoms, leading to a stable molecular structure.
| Atom | Atomic Number | Valence Electrons |
|---|---|---|
| Hydrogen (H) | 1 | 1 |
| Carbon (C) | 6 | 4 |
| Nitrogen (N) | 7 | 5 |
Understanding the Role of Electronegativity
Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a covalent bond. In the HCN molecule, nitrogen is more electronegative than carbon, and carbon is more electronegative than hydrogen. This means that in the bonds between these atoms, the electrons are not shared equally. The more electronegative atom (in this case, nitrogen) pulls the shared electrons closer to itself, resulting in a partial negative charge on the nitrogen atom and a partial positive charge on the hydrogen atom, with carbon having a slight partial positive charge due to the triple bond with nitrogen.
Implications of Valence Electrons for Chemical Reactions
The arrangement and number of valence electrons in the HCN molecule have significant implications for its chemical reactivity. The presence of a triple bond between carbon and nitrogen makes the molecule relatively stable, but it also means that the molecule can participate in addition reactions where the triple bond is broken, and new bonds are formed. The polarity of the molecule, due to the unequal sharing of electrons, also influences its solubility and reactivity with other polar molecules.
In conclusion, understanding the valence electrons in the HCN molecule provides valuable insights into its chemical properties and reactivity. By considering the number of valence electrons in each atom, the type of bonds formed, and the influence of electronegativity, one can predict the behavior of HCN in various chemical environments.
What is the total number of valence electrons in the HCN molecule?
+The total number of valence electrons in HCN can be calculated by adding the valence electrons of hydrogen (1), carbon (4), and nitrogen (5), resulting in a total of 10 valence electrons.
How does the electronegativity of atoms affect the distribution of valence electrons in HCN?
+The electronegativity of nitrogen being higher than that of carbon and hydrogen leads to a polar distribution of valence electrons, with nitrogen pulling electrons closer to itself, thus influencing the molecular polarity and reactivity.
What type of bonds are formed in the HCN molecule, and how do they involve valence electrons?
+In HCN, a triple bond is formed between carbon and nitrogen, and a single bond is formed between hydrogen and carbon. These bonds involve the sharing of valence electrons between the atoms, with the triple bond consisting of one sigma and two pi bonds, which are crucial for the stability and reactivity of the molecule.



