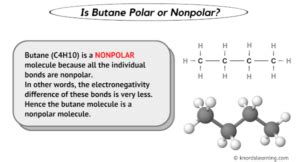
To determine whether C4H10 is nonpolar, we must first understand the molecular structure of this compound. C4H10, also known as butane, is an alkane with a molecular formula consisting of four carbon atoms and ten hydrogen atoms. The structure of butane can exist in two main isomeric forms: n-butane and isobutane (or methylpropane).
Molecular Structure and Polarity
In n-butane, the four carbon atoms are arranged in a linear chain, with each carbon atom bonded to its neighboring carbon atoms through single covalent bonds, and the remaining bonds are filled with hydrogen atoms. In isobutane, three carbon atoms are bonded to a central carbon atom, which is also bonded to a hydrogen atom, and the other carbon atoms are bonded to three hydrogen atoms each.
Polarity of Bonds
The polarity of a molecule is primarily determined by the difference in electronegativity between the atoms in a bond. In the case of butane, the bonds between carbon © and hydrogen (H) are considered to be slightly polar due to the difference in electronegativity between carbon and hydrogen (carbon has an electronegativity of about 2.5, and hydrogen has an electronegativity of about 2.2 on the Pauling scale). However, this polarity is relatively weak.
Shape and Symmetry
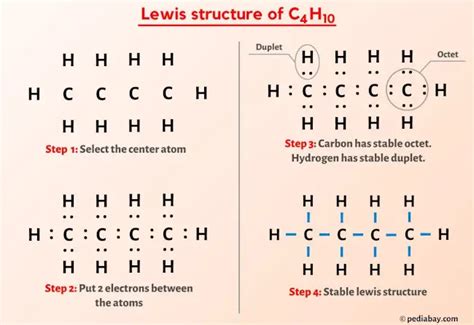
The overall shape and symmetry of a molecule play a crucial role in determining its polarity. A molecule is considered nonpolar if it has a symmetrical shape, meaning that the dipole moments of its bonds cancel each other out. Both n-butane and isobutane have symmetrical shapes. In n-butane, the linear arrangement of carbon atoms with their bonded hydrogen atoms results in a symmetrical molecule where the dipole moments of the C-H bonds cancel out. Similarly, isobutane, despite its branched structure, also exhibits symmetry that leads to the cancellation of dipole moments.
Conclusion on Polarity
Given the symmetrical shapes of both n-butane and isobutane, and considering that the slight polarity of the C-H bonds is cancelled out due to this symmetry, C4H10 (butane) is considered a nonpolar molecule. The lack of a significant net dipole moment in butane is a direct result of its molecular structure, making it nonpolar in nature.
Key Points
- C4H10, or butane, exists in two main isomeric forms: n-butane and isobutane.
- The polarity of a molecule is determined by the difference in electronegativity between bonded atoms and the molecular shape.
- Butane's C-H bonds are slightly polar due to the electronegativity difference between carbon and hydrogen.
- The symmetrical shapes of n-butane and isobutane lead to the cancellation of dipole moments, resulting in nonpolar molecules.
- C4H10 is considered nonpolar due to its symmetrical molecular structure.
Understanding the polarity of molecules like butane is crucial in chemistry, as it influences various physical and chemical properties, such as boiling point, melting point, and solubility. Nonpolar molecules tend to have lower boiling and melting points compared to polar molecules of similar molecular weight, due to the weaker intermolecular forces between nonpolar molecules.
What determines the polarity of a molecule?
+The polarity of a molecule is determined by the difference in electronegativity between the atoms in a bond and the overall shape of the molecule. A molecule with a symmetrical shape, where the dipole moments of its bonds cancel out, is considered nonpolar.
Are all hydrocarbons nonpolar?
+Most hydrocarbons, especially alkanes like butane, are nonpolar due to their symmetrical molecular structures. However, not all hydrocarbons are nonpolar. For example, some hydrocarbons with functional groups that introduce significant polarity, such as alcohols or amines, can be polar.
How does the polarity of butane affect its physical properties?
+The nonpolarity of butane results in weaker intermolecular forces compared to polar molecules. This leads to lower boiling and melting points, as less energy is required to overcome these forces and change the state of the molecule. Additionally, butane is less soluble in water and more soluble in nonpolar solvents due to its nonpolar nature.
In conclusion, C4H10, or butane, is a nonpolar molecule due to its symmetrical molecular structure, which results in the cancellation of dipole moments. Understanding the polarity of molecules is crucial for predicting their physical and chemical properties, as well as their behavior in different environments.