The Bohr model of Silicon is a fundamental concept in understanding the atomic structure of this element. Silicon, with an atomic number of 14, is a metalloid that is widely used in the production of semiconductors, solar panels, and other electronic devices. The Bohr model, developed by Niels Bohr in 1913, provides a simplified representation of the atom, where electrons occupy specific energy levels or shells around the nucleus. In the case of Silicon, the Bohr model helps to illustrate the arrangement of its electrons and the resulting chemical properties.
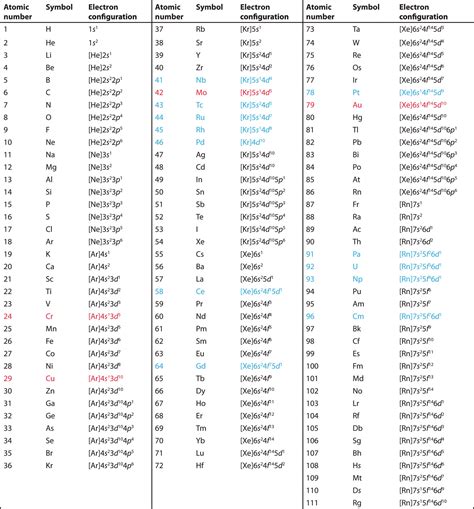
To understand the Bohr model of Silicon, it's essential to start with the basic structure of the atom. The nucleus of Silicon contains 14 protons and 14 neutrons, giving it a total atomic mass of approximately 28 u (unified atomic mass units). The electrons in a Silicon atom are arranged in a specific pattern, with the innermost electrons occupying the lowest energy levels. The electronic configuration of Silicon is 1s² 2s² 2p⁶ 3s² 3p², indicating that the outermost energy level is partially filled with two electrons in the 3p orbital.
Key Points
- The Bohr model of Silicon illustrates the arrangement of electrons in specific energy levels or shells around the nucleus.
- Silicon has an atomic number of 14, with 14 protons and 14 neutrons in its nucleus.
- The electronic configuration of Silicon is 1s² 2s² 2p⁶ 3s² 3p², with two electrons in the outermost 3p orbital.
- The Bohr model helps to explain the chemical properties of Silicon, including its ability to form four covalent bonds.
- Silicon is a metalloid, exhibiting properties of both metals and nonmetals, due to its partially filled outer energy level.
Electronic Configuration and Energy Levels
The electronic configuration of Silicon, as mentioned earlier, is 1s² 2s² 2p⁶ 3s² 3p². This configuration indicates that the innermost energy level (1s) is fully occupied with two electrons, while the subsequent energy levels (2s, 2p, 3s, and 3p) are filled according to the Aufbau principle and the Pauli exclusion principle. The outermost energy level, with two electrons in the 3p orbital, is partially filled, which plays a crucial role in determining the chemical properties of Silicon.
Chemical Properties and Bonding
The partially filled outer energy level of Silicon, with two electrons in the 3p orbital, allows it to form four covalent bonds with other atoms. This is because the two electrons in the 3p orbital can be shared with other atoms, resulting in a stable tetrahedral arrangement. Silicon’s ability to form four covalent bonds is essential for its role in the production of semiconductors and other electronic devices. The chemical properties of Silicon, including its reactivity and bonding patterns, are largely influenced by its electronic configuration and the resulting energy level arrangement.
| Energy Level | Electron Configuration | Number of Electrons |
|---|---|---|
| 1s | 1s² | 2 |
| 2s | 2s² | 2 |
| 2p | 2p⁶ | 6 |
| 3s | 3s² | 2 |
| 3p | 3p² | 2 |
Practical Applications and Real-World Examples
The Bohr model of Silicon has numerous practical applications in various fields, including electronics, renewable energy, and materials science. Silicon’s ability to form four covalent bonds makes it an ideal material for the production of semiconductors, which are used in a wide range of electronic devices, from smartphones to laptops. Additionally, Silicon is used in the production of solar panels, where its ability to form a p-n junction allows for the conversion of sunlight into electrical energy.
Evolution of the Bohr Model and Its Limitations
The Bohr model of Silicon, while providing a simplified representation of the atom, has its limitations. The model does not account for the spin of electrons, the Zeeman effect, or the fine structure of atomic spectra. Furthermore, the Bohr model assumes that electrons occupy specific energy levels, which is an oversimplification of the actual behavior of electrons in atoms. Despite these limitations, the Bohr model remains a fundamental concept in understanding the atomic structure of Silicon and its chemical properties.
What is the electronic configuration of Silicon?
+The electronic configuration of Silicon is 1s² 2s² 2p⁶ 3s² 3p², indicating that the outermost energy level is partially filled with two electrons in the 3p orbital.
Why is Silicon able to form four covalent bonds?
+Silicon is able to form four covalent bonds due to its partially filled outer energy level, with two electrons in the 3p orbital. This allows it to share electrons with other atoms, resulting in a stable tetrahedral arrangement.
What are some practical applications of the Bohr model of Silicon?
+The Bohr model of Silicon has numerous practical applications, including the production of semiconductors, solar panels, and other electronic devices. Its ability to form four covalent bonds makes it an ideal material for these applications.
In conclusion, the Bohr model of Silicon provides a fundamental understanding of the atomic structure and chemical properties of this element. Its electronic configuration, with two electrons in the outermost 3p orbital, allows it to form four covalent bonds and play a crucial role in the production of semiconductors and other electronic devices. While the Bohr model has its limitations, it remains a essential concept in understanding the behavior of electrons in atoms and the resulting chemical properties of elements like Silicon.